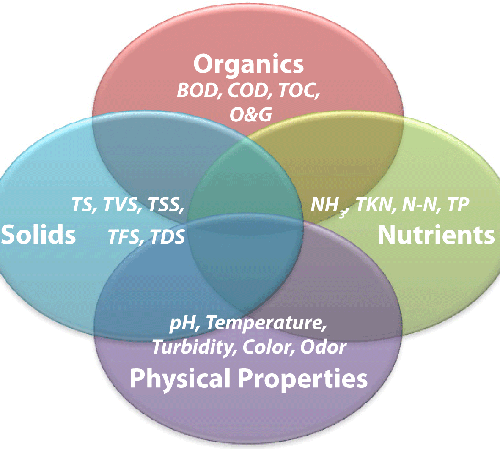
pH is a measure of the concentration of hydrogen ions in the water samples. A high concentration of hydrogen ions causes water to be more acidic and a lower concentration causes the water to be more basic. pH is measured in the field using a pH meter and ranges from 0-14 with the lower values representing higher concentrations of hydrogen ions and the higher numbers representing a lower concentration of the hydrogen ions.
pH is important because increased acidity can stress aquatic organisms, increase the solubility of harmful chemicals in the water, and potentially change the entire ecosystem of a body of water. For instance, if pH increases slightly, there is a chance of eutrophication resulting in increased nutrients and plant life but a decrease in dissolved oxygen.
Effects of pH change usually are demonstrated when levels below 5.0 or above 9.6 are reached. Drastic change can seriously impact aquatic organisms that rely on dissolved oxygen to survive. pH can be affected by many factors, some of which include interactions with surrounding rock, acid rain, wastewater discharge, decomposing pine needles, dissolved carbon dioxide levels, agricultural runoff, industrial runoff and mining operations.
Citation
PH of water. (2013). Retrieved from Fundamentals of environmental measurements website.

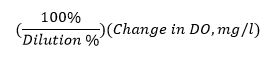 and gives a BOD reading in units of mg/L.
and gives a BOD reading in units of mg/L.